Guest Post from PromoCell: 3 reasons HLA-typed cells give your scientists an immediate advantage
When accuracy meets efficiency
Behind every successful research program is a procurement team that keeps science moving. But even the most capable scientists can only move as fast as their materials allow.
When research depends on immune-relevant human cells used in immunology, oncology, or cell therapy development, waiting weeks for donor typing and verification can derail timelines.
PromoCell provides ready-to-use, HLA-typed human primary cells that are fully characterized, quality-controlled, and available directly through Prendio. These pre-typed cells remove one of the most common bottlenecks in immune-related research: waiting for confirmation that a donor’s immune profile fits the experiment.
For procurement teams, this means fewer sourcing hurdles, faster project starts, and less time lost to re-orders and administrative change requests.
-
Fewer delays because the typing is already done
In many laboratories, scientists must culture primary cells, extract DNA, send samples for HLA typing, and wait for results. Often, the donor profile does not match the study requirements, and the process must start again.
PromoCell eliminates that cycle. Every lot ships pre-typed for key HLA class I and II alleles and is accompanied by complete documentation. Researchers can begin immediately, and procurement can expect predictable delivery and fewer last-minute orders.
Result: faster setup, smoother timelines, and fewer interruptions to project flow.
-
More reliable data and fewer costly repeats
Reproducibility is one of the most persistent challenges in R&D. When donor cells are not properly characterized, immune responses vary and experiments must be repeated.
HLA-typed primary cells enable consistency from the outset. Scientists can design matched test panels or co-culture systems with known immune profiles, improving experimental reproducibility.
The outcome is stronger, more consistent data and reduced waste of reagents and time. For procurement, reliable inputs translate to more efficient spending and better use of research budgets.
-
Built-in confidence for safety and compliance
Cell and gene therapy projects involve rigorous ethical and regulatory oversight. When each donor’s background, including HLA type, is fully documented, preparing for audits and submissions becomes faster and easier.
At PromoCell, we provide HLA typing certificates for all donor lots when the typing is requested at the time of cell acquisition. There is no need for additional supplier follow-up, and all documentation arrives complete with your order.
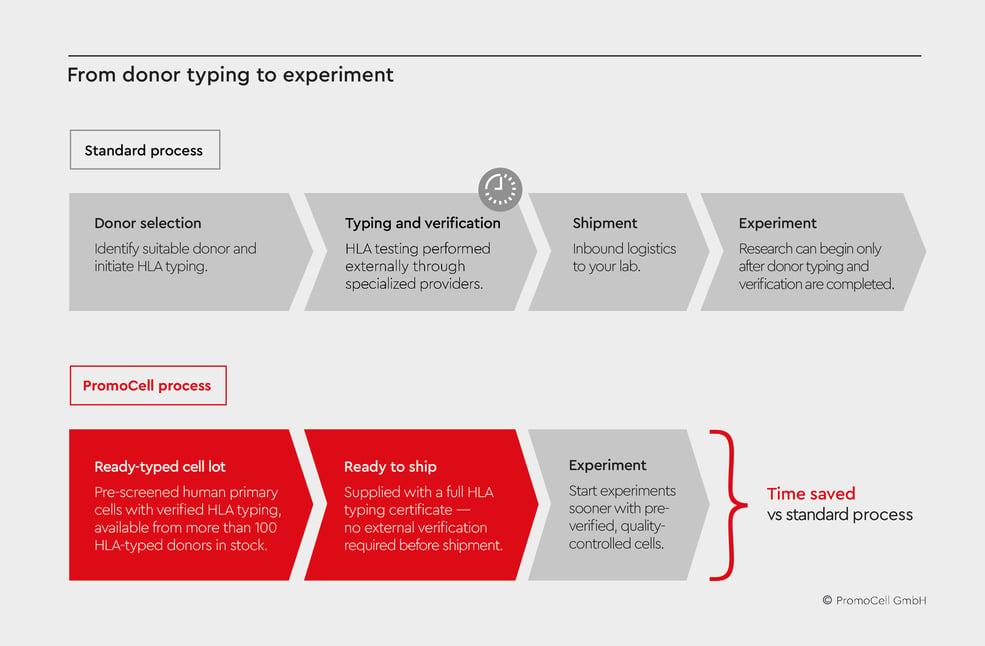
Figure 1: Streamlined immune‑relevant research with HLA‑typed primary cells.
Ready‑to‑use HLA‑typed human primary cells remove the donor typing and verification bottleneck and enable faster, more reliable workflows. Compared with conventional donor typing via third‑party platforms, pre‑typed cell lots accelerate timelines and simplify procurement.
Why your scientists will thank you:
By sourcing HLA-typed cells, your research teams can:
- Start experiments immediately
- Use consistent, human-relevant models
- Validate safety and specificity more efficiently
- Ensure regulatory compliance through complete donor documentation
Because these materials are available directly through Prendio, your purchasing workflow remains seamless, offering the same approval chain and invoice visibility with greater scientific impact per purchase.
About PromoCell
PromoCell GmbH (Heidelberg, Germany) provides high-quality human primary cells and cell-culture media for advanced research and therapeutic development. Its HLA-typed cell portfolio helps labs produce more consistent, relevant results — without delays. Learn more at www.promocell.com.

